Berkeley Drosophila Genome Project
BDGP Resources
In Situ Hybridization Using Digoxigenin Labeled Probes
The Cytogenetics Core performs polytene chromosome in situ hybridizations to polytene chromosomes to serve the needs of the BDGP's various projects. The Core prepares probes and chromosome squashes, carries out hybridizations, and analyzes and records the results of these hybridizations.
The Cytogenetics Core is managed by Todd Laverty and he is responsible for analyzing the results of all hybridizations (his email address is [email protected] or phone 510 643-9780).
Preparation of Chromosome Squashes:
-
For the best results larvae should be grown in well yeasted media at 18°C and care should be taken not to overcrowd the culture
-
On a clean siliconized slide place two drops of 45% acetic acid and a clean siliconized 18 mm square coverslip. On the coverslip place a small drop (2-3 mm) of fixative( 1/2/3 mixture of lactic acid/water/glacial acetic acid)
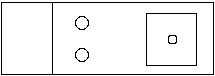
-
In the first drop of 45% acetic acid dissect out the salivary glands from a third instar larva
-
Transfer the glands to the second drop of 45% acetic acid and cut off the anterior ends of the glands. Quite often this will allow the glands to separate from the membrane and the attached fat body. If this does not occur, carefully dissect away the fat body
-
Transfer the "fat free" glands into the fixative and incubate for 4-5 minutes
-
Pick up the coverslip and glands with a clean slide by carefully touching the slide to the drop of fixative.
-
Place the slide, coverslip up, on several sheets of paper towel. With another sheet of paper towel hold the edge of the coverslip to prevent it from moving.
-
Using a stiff, pointed probe, gently press once over the glands. Then gently tap the coverslip, starting over the glands, working your way toward the edge in a spiral pattern.
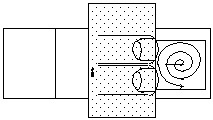
- Turn the slide over so that the coverslip is between the paper towel and the slide. Press gently on one edge of the slide so that the fixative flows to the opposite edge.
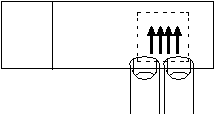
-
Turn the slide back over and again hold the edge of the coverslip with a paper towel
-
Using the probe, gently streak the coverslip with a rapid back and forth motion at one edge and then gradually move to the opposite edge. Often better results are obtained by lightly streaking the slide several times, rather than streaking hard once or twice
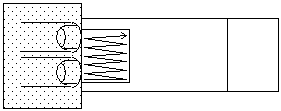
-
Blot off excess fixative and examine under the microscope using phase contrast. If spreading is not sufficient, gently restreak the coverslip. On a good slide the chromosomes should appear low contrast, cytoplasmic debris should be vague, and no fixative should be flowing between the slide and the coverslip.
-
Incubate the slide over night at a temperature between 4° and 18°C. Leaving the slides at room temperature for a couple of hours before incubating them will help when incubating at or near 4°C
-
Place the slides in a dry ice/ethanol bath for 60 minutes. During or before this time cool down some 100% ethanol in a staining dish to -60 to -80°C
-
Remove the slides individually from the dry ice/ethanol bath and quickly flip off the coverslip with a razor blade and place the slide immediately into the chilled ethanol
-
Allow the ethanol to warm up to room temperature, then air dry the slides. Chromosome squashes can be stored at this stage for several months before hybridization
Pretreatment of Chromosomes for Hybridization:
The chromosomes used for hybridization must be non-refractile after ethanol dehydration (A [16]). The chromosomes on the slide should look the same at this point as they did when the slide was made or it is not worth using. In general one slide is enough for a single probe if the chromosomes have good morphology. After the pretreatment the slides should be hybridized within 24 hours.
-
Place the slides in 2X SSC at 65°C for 30 minutes
-
Wash for 2 minutes in 2X SSC at room temperature.
-
Acetylate the slides as follows: Prepare 500 ml of 0.1 M triethanolamine-HCl (1M stock pH 8.0). Add 0.625 ml of acetic anhydride and mix thoroughly. Incubate for 10 minutes. (Some people claim this step is unnecessary, but it has not been tested in this lab.)
-
Wash the slides in 2X SSC for 5 minutes
-
Denature the chromosomes by incubation in freshly prepared 0.07 N NaOH for 3 minutes
-
Wash the slides in 2X SSC two times for 5 minutes each
-
Ethanol dehydrate two times 5 minutes in 70% ethanol and 5 minutes in 95% ethanol. Air dry.
Random Primed DNA Labeling with DIG for Chromosome In Situ's
(Ilaria Rebay, 1996)
2.0 ul 10X DIG Labeling Mixture (Boehringer Cat. #1277 065) 2.0 ul 10X Hexanucleotide Mixture (Boehringer Cat. #1277 081) 15.0 ul DNA + ddH2O 1.0 ul Klenow
20.0 ul Total Reaction Volume
-
Mix DNA (100-500 ng) + ddH2O and boil 5 minutes.
-
Cool immediately on ice (only critical for small probes, cosmids and P1's seem to do fine at room temp.)
-
Add remaining reagents
-
Incubate at room temp. for several hours to overnight (I prefer overnight but if you're in a hurry, 3 hours should give a nice probe)
-
Add 20 ug carrier DNA and 1/10 volume 3M NaOAc. Precipitate with 2.5 volumes EtOH for 1 hr @ -70oC or overnight @ -20oC
-
Spin DNA in microfuge 15 min. Remove EtOH and SpeedVac dry for 2 min
-
Resuspend pellet in 75 ul hybridization buffer.
Hybridization Buffer
0.6 M NaCl 50 mM Sodium phosphate buffer, pH 6.8 1X Denhardt's (0.02% BSA / 0.02% Ficoll / 0.02% polyvinylpyrrolidone) 5 mM MgCl2
Hybridization and Washes:
-
Denature the hybridization probe by boiling for 3 minutes and chill on ice
-
Apply 5 µl of hybridization solution on the slides and distribute the solution over the chromosomes with a 18 x 18 mm coverslip. Try to avoid trapping air bubbles
-
To prevent evaporation of the probe seal the edges of the coverslip with rubber cement
-
Incubate in a moist chamber at 58°C for 12-18 hours
-
Place the slide in a beaker containing 2X SSC and pry off the coverslip and rubber cement with a scalpel blade.
-
Wash the slides 3 times 15 minutes in 2X SSC at 53°C. Proceed directly with the signal detection. It is important not to let the slide dry out during the signal detection procedure.
Signal Detection:
To detect, we use a 1:500 dilution of Anti Digoxigenin-POD (Boehringer Cat. #1207 733).
-
Wash the slides in 1X PBS 2 times for 5 minutes each at room temperature
-
Wash for 2 minutes in 1X PBS/0.1 % Triton-X-100.
-
Rinse in 1X PBS
-
Place the slides horizontally on two 5ml pipettes in a tray lined with moist paper towels. Apply 100µl of a 1:500 dilution of Anti Digoxigenin-POD in 1X PBS, 5mMEDTA. Distribute the solution by placing a 22 x 40 mm coverslip on the slide.
-
Cover the tray with plastic wrap and incubate at room temperature for 30 minutes.
-
Float the coverslip off in a beaker of 1X PBS and repeat steps 1 through 3.
-
Prepare a solution containing 0.5 mg/ml of diaminobenzidine in 1X PBS. Add 1/100 volume of 1% hydrogen peroxide (prepared from a 30 % stock in distilled water). The staining solution should always be prepared fresh. Diaminobenzidine is a carcinogen. Wear gloves when handling the solution. Diaminobenzidine can be inactivated by bleach which should be used liberally when disposing of unused diaminobenzidine and contaminated materials.
-
Place the slides horizontally in a tray and add 50 µl of staining solution onto each slide, cover with a 22 x 40 mm coverslip and incubate at room temperature for 1 - 10 minutes. The staining reaction can be observed by blotting off excess solution and examining the slide under the microscope using a low magnification phase contrast lens (e.g. 16x)
-
Stop the reaction by dipping the slides in distilled water three times and then place them in distilled water until staining
-
Prepare a 4% dilution of Giemsa stain in 10 mM sodium phosphate buffer, pH 6.8. Stain the slides for 30 seconds, rinse in water and allow the slides to air dry. It is important not to overstain the chromosomes for this may obscure the diaminobenzidine precipitate
-
Place a drop of mounting media over the chromosomes and cover with a 22 mm square coverslip
-
Examine the chromosomes using phase-contrast optics. Hybridization signals appear as black bands. Weaker signals are brown
Solutions:
- 10X PBS
1.3 M NaCl 0.07 M Na2HPO4 0.03 M NaH2PO4
- Hybridization Buffer
0.6 M NaCl 50 mM Sodium phosphate buffer, pH 6.8 1X Denhardt's (0.02% BSA / 0.02% Ficoll / 0.02% polyvinylpyrrolidone) 5 mM MgCl2
Reagents and Suppliers:
-
10X DIG Labeling Mixture Cat.# 1277 065 Boehringer Mannheim P.O. Box 50414 Indianapolis, IN 46250-0414 1-800-262-1640
-
10X Hexanucleotide Mixture Cat. # 1277 081 Boehringer Mannheim P.O. Box 50414 Indianapolis, IN 46250-0414 1-800-262-1640
-
Anti Digoxigenin-POD Cat. # 1207 733 Boehringer Mannheim P.O. Box 50414 Indianapolis, IN 46250-0414 1-800-262-1640
-
Diaminobenzidine 3',3' diaminobenzidine tetrahydrochloride Cat. No. D 9015 Sigma Chemical Company P.O. Box 14805 St. Louis, MO 63187 1-800-352-3010
